#AusCT 1Sep17: Clinical Trials With Children and Young People
We all tend to think that the medicines we get prescribed to us have been tested in a way that means they are likely to work for us. As adults, if a medicine is being used for the purpose it was approved, then that is probably a pretty safe assumption.
The same however can’t be said for the treatments given to children and young people. In many cases, there are no clinical trials backing up the dose, effectiveness or safety of medicines in these age groups. This is because for a long time, there was no incentive to run clinical trials with populations aged under 18.
About 20 years ago the EU and US started to look at improving the evidence available to support the use of medicines in the paediatric population. Over the years, there have been various changes in regulation that have increased the level of paediatric clinical trials, and hence medicines with evidence for how they work in children and young people [1,2], but there is still some way to go.
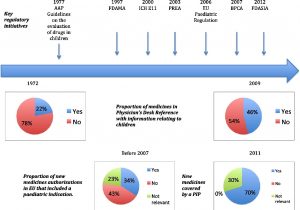
Source: Ref 2
So why do there need to be clinical trials in children and young people? Well, because children are not just ‘little adults’. There are differences in the way they adsorb, distribute, metabolise and excrete medicines, and how medicines affect their bodies. And, the way the medicine is designed to be given to adults may not be suitable for children (for example, if it is a big capsule that a child wouldn’t be able to swallow).
There are plenty of challenges in running clinical trials involving children and young people, including finding enough trial participants, obtaining consent from parents/assent from the young people involved, appropriateness and frequency of study procedures, getting dosing and dosage forms right. It is important though that clinical trials are run for the under 18s, and there is advice out there to help parents who may be considering a clinical trial for their children [For example, Ref 4].
Everyone deserves medicines that are they know are fit-for purpose and safe – including kids. That will only happen, if these age-groups take part in clinical trials.
The next #AusCT twitterchat will explore the issues surrounding clinical trials in the under 18 age group. We would love parents, kids, researchers, trial networks, industry, ethics committee members, consumer groups to all join the discussion and share their experience, questions, advocacy, etc on the following topics that we plan to cover over the hour of the twitterchat:
 T1: Would you enrol your child in a clinical trial? What would influence this decision?
T1: Would you enrol your child in a clinical trial? What would influence this decision?
T2: What are the ethical & practical considerations of running clinical trials for under 18s? How can they be addressed?
T3: What works best, and when, in terms of approaching parents and their children to discuss a trial? What are the typical questions?
T4: What needs to be done to improve the quantity, quality, and practicality of these trials?
T5: What relevant, quality resources/links exist for parents, kids and researchers to learn more about these clinical trials?
I hope you will join the live conversation using the hashtag #AusCT on 1Sep/31Aug depending on your timezone. But if you do miss it, catch up via the link I will post in the comments within a day of the chat.
– Janelle Bowden
2017-09-01 08:00:00
2017-09-01 09:00:00
Australia/Sydney
#AusCT Twitterchat – Clinical Trials With Kids & Young People
Join the live twitterchat using the hashtag #AusCT to share your concerns, thoughts and experience on the topic
Online
Janelle Bowden
admin@research4.me
Further Reading:
1. Zisowsky J, Krause A, Dingemanse J. Drug Development for Pediatric Populations: Regulatory Aspects. Pharmaceutics. 2010;2(4):364-388. doi:10.3390/pharmaceutics2040364.
2. Turner MA, Catapano M, Hirschfeld S, Giaquinto C, Paediatric drug development: The impact of evolving regulations, Advanced Drug Delivery Reviews. 2014;73:2-13. http://dx.doi.org/10.1016/j.addr.2014.02.003.
3. European Medicines Agency (EMA): Paediatric Regulations
4. US Food and Drug Administration (FDA): Would Your Child Benefit from a Clinical Trial?




Janelle Bowden
September 5, 2017 at 10:18 pmFor those that missed the chat, feel free to catch up on the summary here: https://storify.com/Res4Me/ausct-1sep17-chat-summary-involving-kids-in-clinic
Riya Patel
December 27, 2017 at 7:06 pmGreat Blog !! A vital information is being shared by you via this blog regarding “Clinical Trials With Children and Young People”. The information was really helpful it’s really informative and and covers all the aspects. I want to encourage yourself to continue your great writing. I think-Clinical trials are research studies involving people. They test whether particular treatments are safe and how well they work. We need to know: Does a treatment work? Does it work better than other treatments? Does it have any side effects? Clinical trials are designed to answer these questions to improve the health and quality of life for patients, including children. Until well-designed trials have been carried out, we simply do not have enough evidence to know if a treatment is both effective and safe. Without trials, there is a risk that people will be given treatments which do not work and which may be harmful.