Why, What, When, Who, How to Inform the Public About Clinical Trials: #AusCT chat 3Feb17
The past weekend I was reminded of how clinical trials can help change lives. I was lucky enough to get away with my family for a long weekend break by the seaside. One evening, as my husband was cooking our dinner on a BBQ beside the beach, we got to chatting to the family using the neighbouring BBQ. They mentioned their 5 year old was starting school this week, but that it might never have happened. A year ago they got the devastating news that their then 4yr old had leukemia. As I looked at my 3yr old playing on the nearby monkey bars, I couldn’t imagine how that would have felt as a parent.
The family have spent a lot of time in hospital over the past year, and had great admiration and praise for the children’s oncology team. Their child was enrolled in a clinical trial, and so far, so good…he starts school this week.
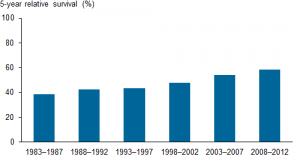
5-year relative survival from leukaemia, 1983–1987 to 2008–2012 Source: AIHW analysis of the Australian Cancer Database
5-yr survival rates for leukaemia have increased in Australia from being less than 40% in 1983-87 to 58% in 2008-12, according to the Australian Institute of Health and Welfare website. We have families such as the one I met, willing to go into clinical trials to evaluate what works, to thank for the almost doubling of leukaemia survival rates over this period.
Our short conversation ended as each of our dinners were cooked, but I was left wondering about what they knew about clinical trials before being approached, and how they went about evaluating if it was right for their child. This conversation was perfectly timed to inspire my thinking for the upcoming #AusCT chat this Friday 3 Nov, at 12.30pm Sydney, Australia time.
As a clinical trials professional, I know how to go about finding trials, assessing them, the process for getting involved, my rights and protections as a potential participant, what I should expect in terms of information and communication with the research team, the potential risks and benefits, and the like. But this is not common knowledge for the general public.
When is the right time to provide general information about clinical trials to the general public? Is it just as they receive a diagnosis, or when their doctor happens to have a trial open they might be suitable for, or should it be part of the baseline education we receive at school given one day we will likely all interact with the health system as a patient?
What information do the public want to know? Is it enough to know that clinical trials exist and that if needed, people can search online to find them? Or should we be educating on the role of clinical trials in developing better care and treatments, they types of trials, the benefits and risks, etc, and the active role the public can play in creating better, faster trials that are relevant to patient needs?
How should education about clinical trials be delivered? By leaflets, posters, bus advertising, online tutorials, videos, peers, face-to-face teaching, government or private websites, or all/other methods?
Who should provide education and information about clinical trials? Physicians, healthcare institutions, clinicians, school teachers, former trial participants, governments, PR and health communications experts, researchers and research organisations, consumer groups, private companies, etc? How do the public decipher who is providing accurate information?
For me, the given is Why we should talk about clinical trials… because they help us know what works and is safe for patients in the future, and may just help us in the process.
 I’d like to know what you think about these questions, so I hope you will join us in discussing them on the next #AusCT twitter chat, Friday 3 Feb, 12.30pm Sydney Australia time.
I’d like to know what you think about these questions, so I hope you will join us in discussing them on the next #AusCT twitter chat, Friday 3 Feb, 12.30pm Sydney Australia time.
For information, the #AusCT will be on the first Friday of each month, at 12.30pm Sydney, Australia time during 2017. And in case you missed the last chat, a summary is posted here.




Janelle Bowden
February 3, 2017 at 2:37 pmFor those that missed this chat, the summary is posted here: https://storify.com/JanelleBowden/ausct-3feb17-wrap. Next chat scheduled for Fri 3 Mar, 12.30pm AEDT, and will be guest moderated by Melanie Gentgall, Praxis Australia. The topic will be around ethical challenges/issues in clinical trials.